The ions in an ionic compound stack up in a regular repeating pattern called a ____ in order to maximize attraction and minimize repulsion White Most ionic compounds that do not contain transition metals are what color. Simple acids known as binary acids have only one anion and one hydrogen.
5 7 Naming Ionic Compounds Chemistry Libretexts
Na 2 O 2 2H 2 O 2NaOH H 2 O 2.
. They are very hard somewhat brittle solids with extremely high melting points higher than 1000 C or 1800 F. From the above example ionic compounds can be defined as the compounds formed by the transfer of electrons between metals and non-metals. Unlike ionic compounds they do not dissolve in water nor do they conduct electricity.
Due to the presence of oppositely charged ions ionic compounds are held strongly by the electrostatic force of attraction. Nomenclature of Ionic and Covalent Compounds. It was noted that the composition of dispersed phase particles in a liquid dispersion medium should necessarily include adsorbed counterions rigidly bound to these particles.
Iodine is a chemical element with the symbol I and atomic number 53. The peroxide anion is weakly bound to the cation and it is hydrolysed forming stronger covalent bonds. The heaviest of the stable halogens it exists as a semi-lustrous non-metallic solid at standard conditions that melts to form a deep violet liquid at 114 degrees Celsius and boils to a violet gas at 184 degrees CelsiusThe element was discovered by the French chemist Bernard Courtois in 1811 and was named two.
The existing concepts of the ionic micelle structure were specified. It correlates the chemical profile and the bioactivity pattern of plant extracts to guide the isolation of compounds and to identify new or already known bioactive constituents at an early stage dereplication Tawfike et al 2013. There may or may not be more than one of each element.
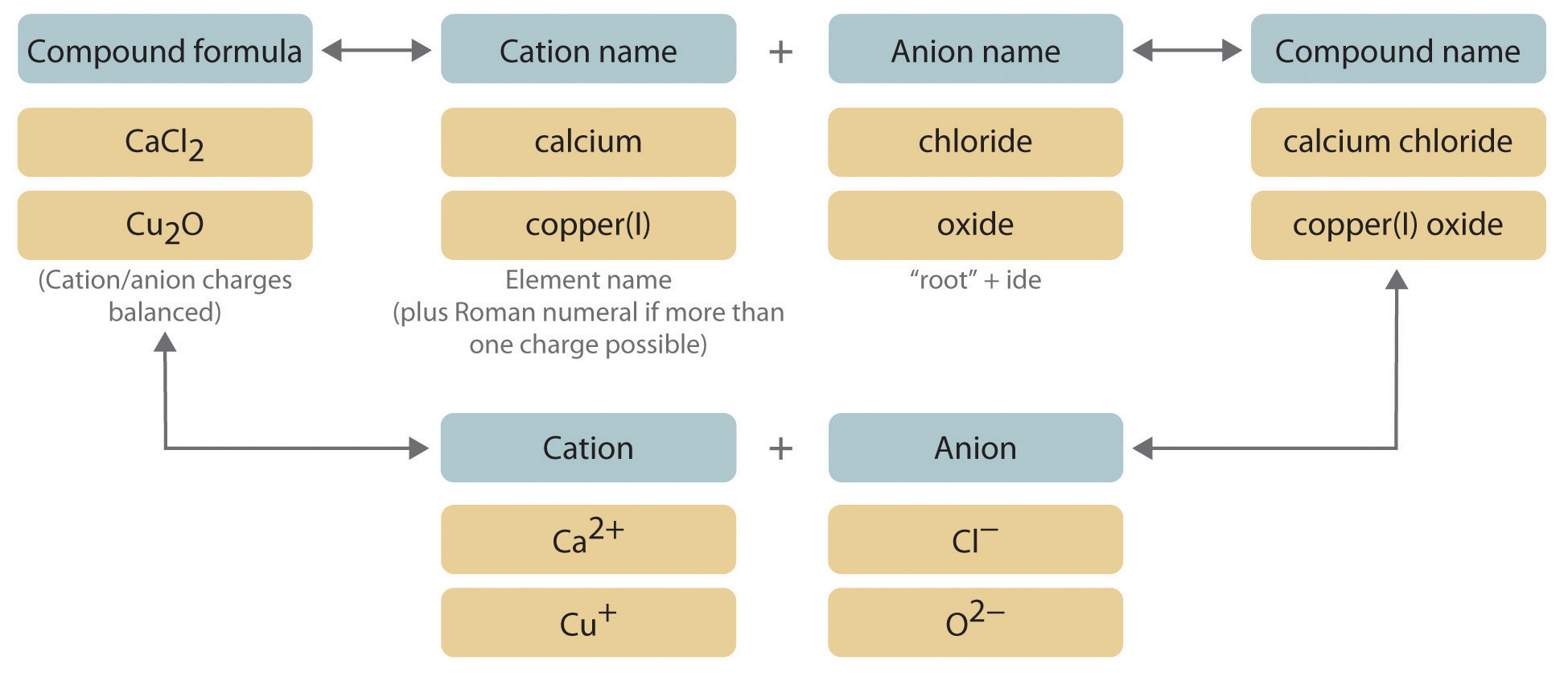
A binary compound is a compound formed from two different elements. A diatomic compound or diatomic molecule contains two atoms which may or may not be the same. The bond formed between them is known as the ionic bond.
Binary ionic compounds are salts which consist of only 2 elements. The binary compound list is mentioned in the table below. These anions usually have the ending -ide As acids these compounds are named starting with the prefix hydro- then adding the first syllable of the anion then the suffix -ic For example HCl which is hydrogen and chlorine is called hydrochloric acid.
The alkali metal peroxides are ionic compounds that are unstable in water. By numerical solution of the Poisson equation for the two most often used approximations the PoissonBoltzmann PB. Nomenclature of common acids.
The other oxygen compounds are also unstable in water. 2KO 2 2H 2 O 2KOH H 2 O 2 O 2 Li 2 O H 2 O 2LiOH. Here both elements are ions an anion which has a negative charge and a cation which has a positive charge.
The goal of metabolomics in general is to analyze all secondary metabolites in a sample qualitatively and quantitatively. Wolfender et al 2014. Binary Ionic Compounds Containing a Metal and a Nonmetal.
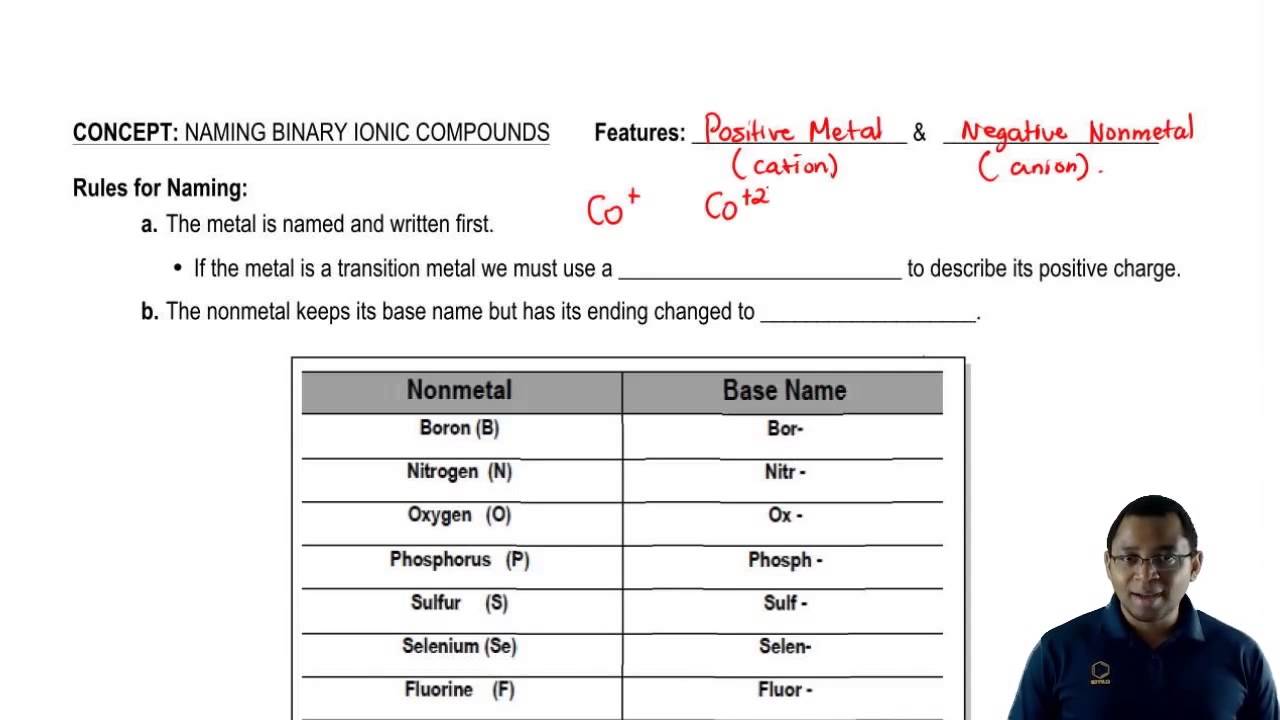
Rules For Naming A Binary Ionic Compound Youtube

Forming And Naming Ionic Compounds Type 1 And 2 Binary Compounds Ppt Download

Solved Part Ii Binary Ionic Compounds Containing Chegg Com
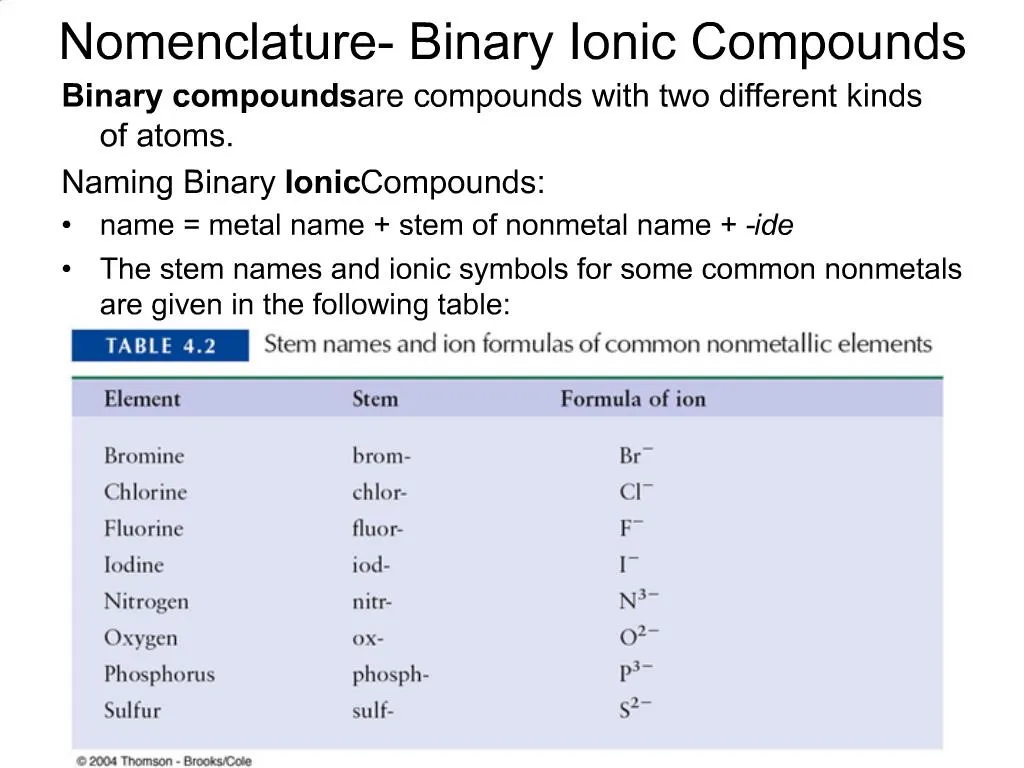
Ppt Nomenclature Binary Ionic Compounds Powerpoint Presentation Free Download Id 721132



0 comments
Post a Comment